

Cancel-You need to just reach on the date you want to cancel and click on the cancel button.

Just log in to account, click on the session date, reschedule it and then click OK. Reschedule- You can reschedule your session at least 24 hours prior to your former schedules session from your account. Once the link starts appearing in the bar, click on launch class and we are directed to WebEx whiteboard. Virtually all elements exist as mixtures of isotopes, so atomic masses may vary significantly from whole numbers. You will receive a link 15 minutes prior to your fixed scheduled session. The atomic mass of boron is calculated similarly to what we did for our hypothetical example, but the percentages are different: Thus, we use 10.8 u for the atomic mass of boron.

Carbon exists on Earth as about 99 12 C and about 1 13 C, so the weighted average mass of carbon atoms is 12.01 u. Similar average atomic masses can be calculated for other elements. Then you can select date time and days according to your requirement and then confirm it. The atomic mass of boron would be calculated as (0.199 × 10.0 u) + (0.801 × 11.0 u) 10.8 u. This is the reason why the mass of a boron atom is 10.81 u despite not having a boron atom with a mass of 10.81 u. Log in to your account and you will see a schedule class button on the right corner. The atomic mass (m a or m) is the mass of an atom. For Boron, Number of Protons 5 Number of Neutrons 6 Thus, Atomic Mass of Boron 5 + 6 11 In absolute terms, the average atomic mass of Boron is 10.81 amu. Scent, Tie, Vase, Crystal, Mango Fetch the Cobra Night by Current Zendaya Atomic Mass of any substance is the sum of the number of protons and the number of neutrons in the nucleus of that atom. Nation Mgell Always Sign Patrol Safety Clause Agreement King of Canada These elements can be remembered by this line Naturally occurring boron is 80.20 boron-11 (atomic mass.
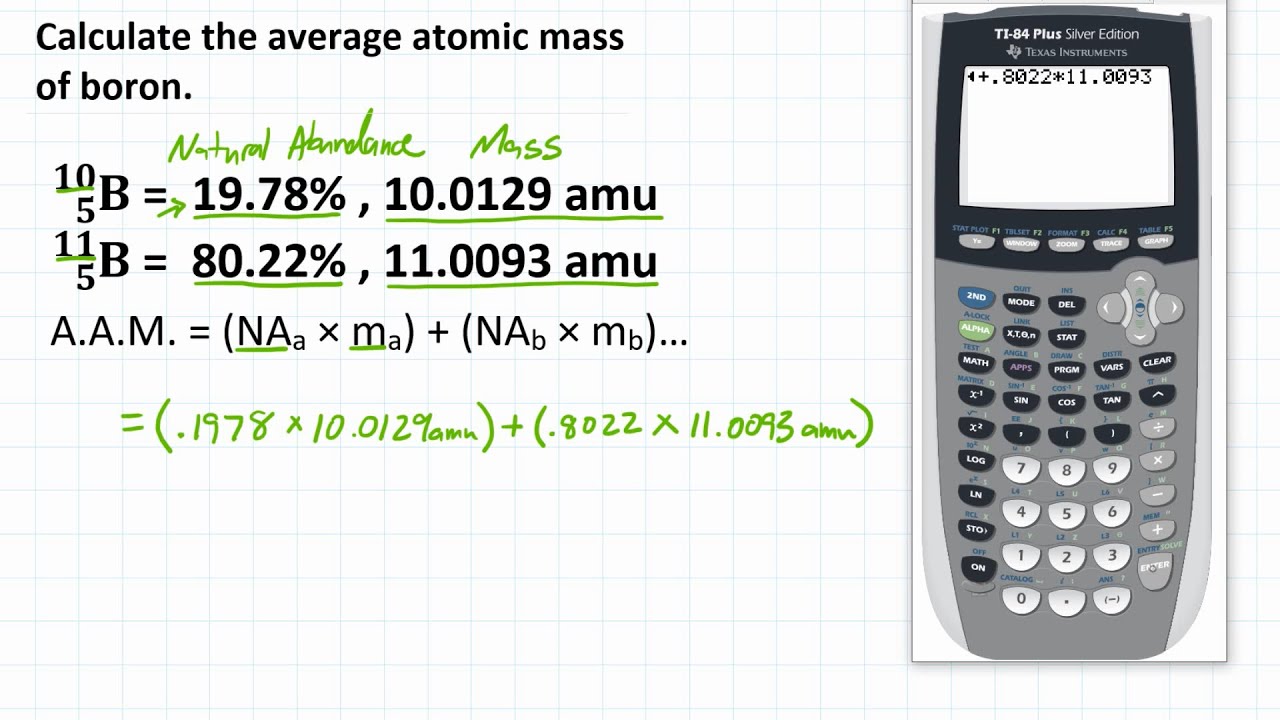
Harley Health Like Beautiful Body of Cheetah Name Opposite Falcon Nest. Only two isotopes of boron (B) occur in nature their atomic masses and abundances are given in the following table. If boron has an atomic mass of 10.81 amu, which isotope occurs in greater abundance chemistry. These elements can be remembered by this line: Their respective masses are 10.01294 u and 11.00931 u, and the atomic mass of boron is 10.811 u. The mass of the B isotope is 10.0129 amu whereas the mass of B is given to be 11.0093 amu. High-pressure SF 6 gas is used in place of older oils that may have contaminants that are not environmentally friendly (part (c) in the accompanying figure).If we are talking about the first 30 elements then the periodic table starts with Hydrogen and ends at Zinc that is an element with atomic number 30. The atomic mass of boron (B) is given to be 10.81 amu. Sulfur hexafluoride also has another interesting use: a spark suppressant in high-voltage electrical equipment. Miller, Boron determination in biological materials by inductively coupled plasma atomic emission and mass spectrometry: effects of sample dissolution methods, Fresenius Journal of Analytical Chemistry, vol. (c) A high-voltage electrical switchgear assembly that would be filled with SF 6 as a spark suppressant. Boron element is widely distributed in different geologic bodies. (a) Properly protected workers clear out the Tokyo subway after the nerve toxin sarin was released. \): Sarin and Sulfur Hexafluoride © Thinkstock.


 0 kommentar(er)
0 kommentar(er)
